协会服务热线
010-6226-7180

时间/地点:2018年9月1日(Sep 1)/会议室5(M5)
内容介绍:
随着《化学药品与弹性体密封件相容性研究技术指导原则(试行)》的发布实施,美国PQRI关于大容量注射剂的安全阈值也最新确定,国内外相关法规进入新一轮更新中。“相容性研究理论与实践”专场培训会将邀请国内外权威人士齐聚一堂,分享相容性领域的最新法规以及实践经验分享。
此次培训,您将了解掌握以下领域的最新研究动态:《化学药品与弹性体密封件相容性研究技术指导原则(试行)》条款解读、药用弹性体密封件提取研究中未知物分享、药用玻璃耐水性测试最新研究及USP<660>最新进展、相容性研究试验方法的确定、基于风险角度的可提取物和浸出物评价、生物制品相容性研究注意事项、药包材毒理学风险评估及应用等内容。
会议日程:
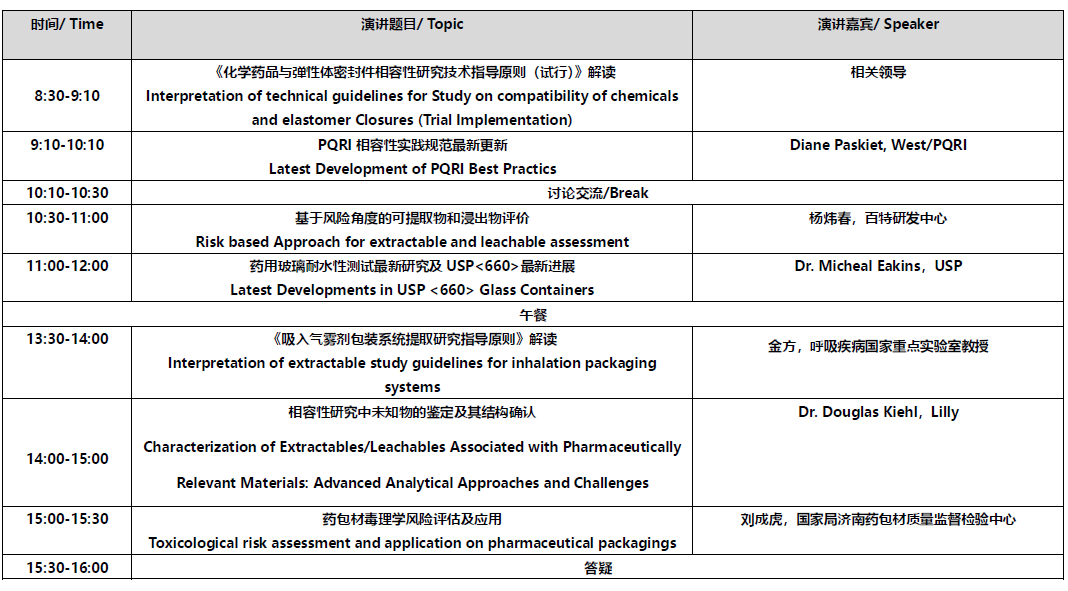
会议收费:500元/人
演讲嘉宾:
 Michael N. Eakins
Michael N. Eakins
Michael was Vice-Chair of the USP Packaging, Storage and Distribution Expert
Committee in the 2005-2010 and 2010-2015 cycles and is currently a member of
the USP Packaging and Distribution Expert Committee for the 2015-2020 cycle. He is an active member of the Parenteral Drug Association, being the co-chair of the Glass Defects Task Force that revised Technical Report 43 and was a member of the Elastomers and Seals Defects Task Force responsible for Technical Report 76 published in 2016. He obtained his Ph.D. from London University and has contributed to over 60 publications and 8 USA patents.
 DIANE PASKIET
DIANE PASKIET
Diane Paskiet has over twenty years of experience with qualifying packaging and
delivery systems for use with pharmaceutical products. She is Currently Director of
Scientific Affairs at West Pharmaceutical Services where she is involved in science and regulatory programs associated with safety and compatibility of packaging systems. Previous to this role she was in charge of site operations for West-Monarch Analytical Laboratories. She is a co-recipient of the United States Pharmacopeia (USP) award for Innovative Response to a Public Health Challenge and 2019 Vice Chair of Packaging Storage and Distribution Committee. She also serves as Vice Chair of Product Quality Research Institute (PQRI) Development Technical Committee (DTC) and Chair of Parenteral and Ophthalmic Drug Product Leachables and Extractables Working Group.Ms. Paskiet is also on the faculty of the Parenteral Drug Association Training Institute and author/co-author of papers related to pharmaceutical packaging.
 Douglas Kiehl
Douglas Kiehl
Douglas Kiehl is a Research Advisor at Eli Lilly and Company and leads the
Spectroscopy and Extractables/Leachables team. He is responsible for Lilly’s
global E&L strategy supporting development and qualification of container/closure
and manufacturing systems and drug delivery devices. His team’s responsibilities include performing structural characterization of process impurities, related substances, degradation products and contaminants across development and commercialization phases for the small and large molecule portfolios. Mr. Kiehl has over 35 years' experience with application of advanced mass spectrometry in characterization of diverse chemical entities, 23 years of which are in the Pharmaceutical Industry. He is a member of the USP Expert Panel on Biocompatibility of Materials Used in Packaging Systems, Medical devices and Implants Expert Panel and the USP Expert Panel on Elastomeric Closure for Injections, and represents his company on the ELSIE (Extractables/Leachables Safety Information Exchange) Board of Directors and AAPS Impurities Steering Committee. His research interests include the development and application of advanced mass mapping and visualization techniques for the rapid characterization of highly diverse and complex molecular mixtures.
 杨炜春
杨炜春
杨炜春博士目前在百特研发中心任可提取物和浸出物部门经理。在相容性研究,分析化学,
生物芯片,新药研发,食品科学与安全等领域有极深入的研究。有近20年的分析化学经
验,了解国内外本行业的最新动态。在国内外期刊上发表30余篇论文,其中国际刊物20余篇。
 刘成虎
刘成虎
国家局济南药包材质量监督检验中心生物室副主任,长期从事医疗器械及药包材生物学评
价工作。
 金方
金方
1987年毕业于中国药科大学药剂学专业,上海医药工业研究院药剂学硕士、博士,美国
VCU大学博士后,享受国务院特殊津贴,现为呼吸疾病国家重点实验室特聘教授、PI,健康
元药业集团首席科学家。长期从事药物新剂型及其产业化研究,曾任上海医药工业研究院“创新药物与制药工艺国家重点实验室”副主任、上海呼吸系统药物工程技术研究中心主任。第九、十、十一届国家药典委员会委员,2006年当选为人事部“新世纪百千万人才工程”国家级人选;2007年被评为上海市“三八红旗手”;2008年当选“上海市优秀学科带头人”; 2010年被评为“九三学社中央优秀社员”;2012年获“全国优秀科技工作者”称号。
|
|
|
|